亚盛医药(6855.HK)今日宣布,公司临床开发阶段1类新药Bcl-2抑制剂APG-2575日前接连获得美国FDA两项临床试验许可,将分别开展 作为单药或联合治疗复发/难治慢性淋巴细胞白血病(Chronic Lymphocytic Leukemia ,r/r CLL)/小淋巴细胞淋巴瘤(Small Lymphocytic Lymphoma ,r/r SLL)的Ib/ II期研究; 作为单药或联合治疗华氏巨球蛋白血症(Waldenström Macroglobulinemia,WM)的Ib /II期研究。
此外,APG-2575日前还在中国获得国家药品监督管理局药物审评中心(CDE)临床许可,将开展单药或联合治疗复发/难治性急性髓系白血病(Acute myeloid leukemia,r/r AML)的Ib期研究。
APG-2575
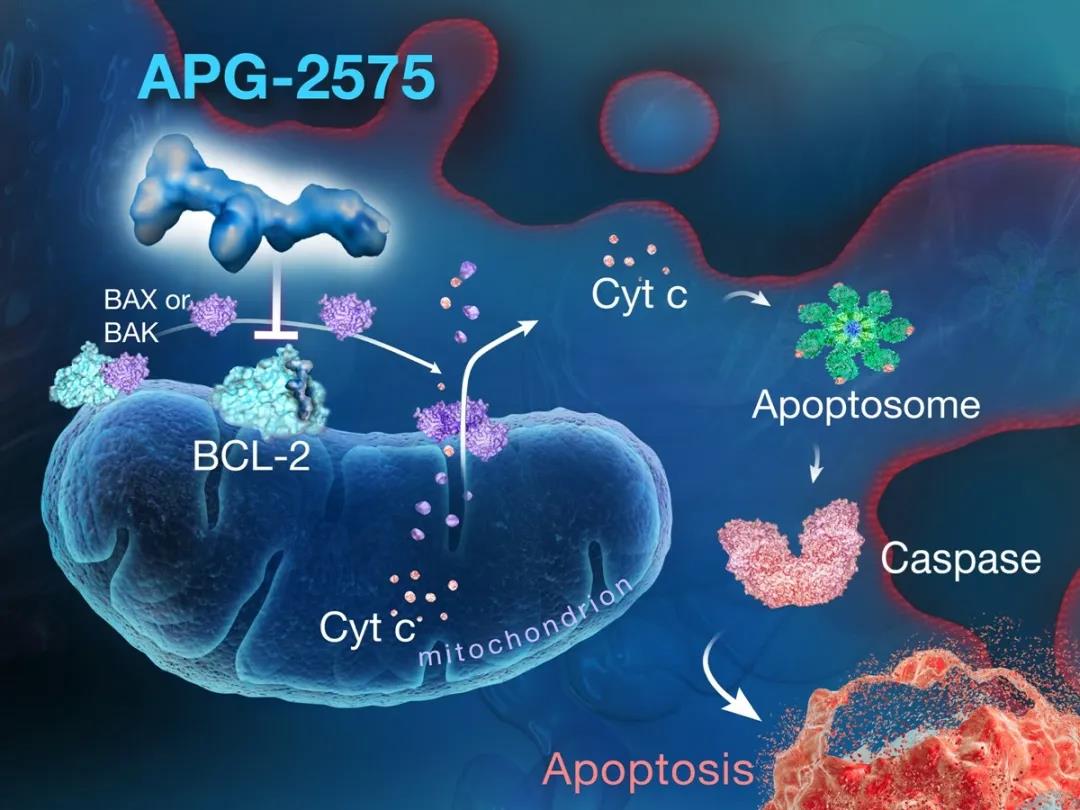
APG-2575是亚盛医药临床开发阶段的新型口服Bcl-2选择性小分子抑制剂,通过选择性抑制Bcl-2蛋白来恢复肿瘤细胞程序性死亡机制(细胞凋亡),从而杀死肿瘤,拟用于治疗多种血液恶性肿瘤。以Bcl-2家族蛋白为靶点的药物研发多年来被证实是极其困难的,但2016年4月在美国成功上市的Bcl-2选择性抑制剂Venclexta为该靶点药物的开发提供了强有力的临床验证依据。APG-2575则是在全球层面继Venclexta之后罕有的进入临床开发阶段的Bcl-2选择性抑制剂。APG-2575去年在中国启动血液肿瘤的I期临床研究,成为首个进入临床阶段的国产Bcl-2选择性抑制剂。
此前,APG-2575针对治疗血液肿瘤的I期临床试验已在美国和澳大利亚启动,迄今未观察到任何剂量限制性毒性(DLT)以及Bcl-2抑制剂常见的肿瘤溶解综合征(TLS),显示了APG-2575在临床试验中的良好的安全性。正是基于APG-2575良好的临床前数据及早期临床数据,亚盛医药针对APG-2575在联合CD20 单抗、BTK抑制剂等药物治疗CLL/SLL、WM、AML的Ib/ II期研究接连获得中美两国药监部门的临床许可。
该项研究为全球多中心、开放性Ib/ II期剂量疗效探索研究,旨在评估APG-2575单药或者联合rituximab/acalabrutinib治疗复发难治CLL/SLL患者的安全性、耐受性,并初步评估有效性。
CLL/SLL是一种成熟 B淋巴细胞克隆增殖性肿瘤,是欧美发达国家发病率最高的成人白血病,约占所有白血病的 30%,年发病率2-6/10万人,65岁以上高达12.8/10万人1。CLL发病率在亚洲国家虽较欧美低,但近年呈上升趋势,具有发病年龄低、侵袭度高等特点。尽管一线方案明显提高CLL患者初治缓解率,但需长期服药控制病情,一旦复发往往预后极差。而近期研究发现,BTK抑制剂依鲁替尼(ibrutinib)联合Bcl-2抑制剂治疗CLL具有深度反应率高的优点,甚至有可能变长期治疗为有限周期治疗,为CLL患者临床治愈和停药提供可能。这无疑也为APG-2575的联合用药探索提供了基础。
该项研究为全球多中心、开放性Ib/ II期剂量疗效探索研究,旨在评估APG-2575单药或者联合依鲁替尼(ibrutinib)/利妥昔单抗(rituximab)治疗WM患者的安全性、耐受性、PK特征及初步的疗效观察。
WM是一种少见的惰性成熟B细胞淋巴瘤。目前指南推荐的WM的治疗方案客观缓解率(ORR) 可达到80%,但是很好部分缓解(VGPR)以上的较深缓解率很低(20%左右或更低),较多患者最终会复发或进展。同时,WM的中位发病年龄在70岁左右,患者身体状态常常不能够耐受强烈治疗。因此WM治疗效果的提高是临床迫切需要解决的问题2。
临床前数据证实,APG-2575可以克服依鲁替尼(ibrutinib)不敏感的耐药WM模型,同时在非霍奇金淋巴瘤(NHL)包括滤泡淋巴瘤、弥漫大B细胞淋巴瘤以及WM模型中,与依鲁替尼(ibrutinib)具有显著的协同效果。
该项研究为多中心、开放性Ib期剂量疗效探索研究,旨在评估APG-2575单药或者联合化疗治疗AML患者的安全性、药代动力学。
AML是中国最常见的白血病,发病率约为1.62 ~ 2.32人/10万人,且主要为一种老年患者疾病,诊断时的中位年龄为67岁。AML的发病率随着年龄的增加而增加,在≥ 80岁达到最高值,发病率为22.5人/10万人。
虽然AML的治疗手段不断进步,但基础治疗模式近年来一直没有变化,高强度化疗和异基因造血干细胞移植是AML的主要治疗手段和模式。即使如此,AML的长期生存率仍然很低,有多达40%的新诊断AML患者在初始诱导缓解治疗期间不能达到完全缓解(CR)而成为难治病例,或在获得CR后6个月内复发。这与AML发病急、复发率高、基因突变复杂等特点密切相关。另外,患者老龄化趋势也是影响AML治愈率的原因之一,许多老年患者无法耐受强化疗3。
临床前证据显示,APG-2575单药具有一定的抗AML效果;同时,APG-2575与各种化疗的联合对于治疗AML有很强的协同作用。值得一提的是,Bcl-2抑制剂为基础的联合化疗还具有较好的安全性和耐受性,有望为老年AML和不耐受强化疗的AML患者提供新的治疗选择。
“APG-2575是公司细胞凋亡产品管线的重要临床开发产品,是首个进入临床的国产Bcl-2选择性抑制剂。基于此前良好的临床前数据及早期临床数据,APG-2575接连获得中国CDE和美国FDA多项Ib/ II期临床试验许可,也正是我们全球化临床开发策略全面推进的体现。联合用药是肿瘤治疗未来发展趋势,希望我们的临床试验能更早、更快让复发难治的血液肿瘤患者受益。”
亚盛医药首席医学官翟一帆博士
关于亚盛医药
亚盛医药(6855.HK)是一家立足中国、面向全球的处于临床阶段的原创新药研发企业,致力于在肿瘤、乙肝及与衰老相关的疾病等治疗领域开发创新药物。公司拥有自主研发的蛋白-蛋白相互作用靶向药物设计平台。2019年10月28日,亚盛医药在香港联交所主板成功上市。
亚盛医药研发产品管线主要专注细胞凋亡路径关键蛋白的抑制剂,通过抑制BCL-2、IAP 或 MDM2-p53 等,重启肿瘤细胞的凋亡程序;第二代和第三代的针对癌症治疗中出现的激酶突变体的抑制剂等。公司现有8个1类新药已进入到临床开发阶段,正在中国、美国及澳大利亚开展28项I/II期临床试验。
参考文献
1. Slager SL, Benavente Y, Blair A, et al. Medical history, lifestyle, family history, and occupational risk factors for chronic lymphocytic leukemia/small lymphocytic lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;48:41-51.
2. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN guidelines®). Waldenström macroglobulinemia/ lymphoplasmacytic lymphoma. Version 1.2020 December 6, 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/waldenstroms.pdf. Last accessed March 8, 2019.
3. Thol F, Schlenk RF, Heuser M, Ganser A. How I treat refractory and early relapsed acute myeloid leukemia. Blood 2015;126:319-27.
前瞻性声明
本文所作出的前瞻性陈述仅与本文作出该陈述当日的事件或资料有关。除法律规定外,于作出前瞻性陈述当日之后,无论是否出现新资料、未来事件或其他情况,我们并无责任更新或公开修改任何前瞻性陈述及预料之外的事件。请细阅本文,并理解我们的实际未来业绩或表现可能与预期有重大差异。本文内所有陈述乃本文章刊发日期作出,可能因未来发展而出现变动。
Ascentage Pharma Announces Clearances for Three Phase Ib/II Clinical Studies of APG-2575 in China and the U.S., Beginning Simultaneous Clinical Development in Three Hematologic Malignancy Indications
Ascentage Pharma (6855.HK), a globally focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases, today announced that two clinical studies of APG-2575, a novel Bcl-2 selective inhibitor being developed by Ascentage Pharma, have recently received clearances from the U.S. Food and Drug Administration (FDA). These studies include one Phase Ib/II trial of APG-2575 as a single agent or in combination for the treatment of relapsed/refractory chronic lymphocytic leukemia (r/r CLL) or small lymphocytic lymphoma (r/r SLL); and another Phase Ib/II trial of APG-2575 as a single agent or in combination for the treatment of Waldenström macroglobulinemia (WM) (MAPLE-1). Furthermore, following recent approval from the Center for Drug Evaluation (CDE) of the China National Medical Products Administration (NMPA), the company will initiate a Phase Ib study of APG-2575 as a single agent or in combination for the treatment of relapsed/refractory acute myeloid leukemia (r/r AML) in China.
APG-2575 is a novel, orally administered Bcl-2 selective inhibitor being developed by Ascentage Pharma. APG-2575 is designed to treat a variety of hematologic malignancies by selectively blocking Bcl-2 to restore the normal apoptosis process in cancer cells. It has proved to be difficult to develop drugs targeting the Bcl-2 family of proteins. However, the marketed Bcl-2 inhibitor Venclexta® (venetoclax), approved by the U.S. FDA in April 2016, has validated the clinical basis for further development of new agents in this class. Globally, Ascentage Pharma’s APG-2575 is one of the few Bcl-2 selective inhibitors in active clinical development. With the Phase I study of APG-2575 in hematologic malignancies initiated in China last year, APG-2575 represents the first China-made Bcl-2 selective inhibitor to enter clinical trials.
A Phase I study of APG-2575 in hematologic malignancies was previously commenced in the U.S. and Australia. No dose-limiting toxicity or tumor lysis syndrome (commonly associated with other Bcl-2 inhibitors) has been observed, suggesting that APG-2575 may have a favorable safety profile. Based on preclinical and early-stage clinical data, Ascentage Pharma has been granted a number of clearances in China and the U.S. for the Phase Ib/II studies of APG-2575 in combination with anti-CD20 monoclonal antibodies, Bruton’s tyrosine kinase (BTK) inhibitors, and other therapeutic agents in CLL/SLL, WM, and AML.
◆A Phase Ib/II study of APG-2575 as a single agent or in combination with rituximab or acalabrutinib for the treatment of patients with r/r CLL/SLL
This global multicenter, open-label Phase Ib/II dose-escalation and dose-expansion study is designed to evaluate the safety, tolerability, and anticancer activity of APG-2575 as a single agent or in combination with rituximab or acalabrutinib in patients with r/r CLL/SLL.
CLL/SLL is a hematologic malignancy caused by mature B-cell neoplasms and is the most common form of adult leukemia in North America and Europe, accounting for about 30% of all new leukemia cases, with an annual incidence of 2 to 6 cases/100,000, and 12.8 cases/100,000 in the population aged 65 or older1. The incidence rate of CLL in Asia is lower than in North America and Europe but has been increasing in recent years, with more patients developing the condition at younger ages and displaying greater disease invasiveness. Despite significant initial responses to current first-line treatments, many patients with CLL need ongoing treatment to maintain these responses, and relapse often portends a poor prognosis. Recent studies in CLL showed that combining BTK inhibitor ibrutinib with another Bcl-2 inhibitor can deepen responses and even shorten cyclic treatment, making it possible for patients to achieve complete remission and therefore discontinue treatment. These findings have provided a compelling rationale for exploring APG-2575 in combination with other therapeutic agents.
◆A Phase Ib/II clinical study of APG-2575 as a single agent or in combination with ibrutinib or rituximab for the treatment of patients with WM
This is a global multicenter, open-label Phase Ib/II dose-escalation and dose-expansion study designed to evaluate the safety, tolerability, pharmacokinetics (PK), and efficacy of APG-2575 as a single agent or in combination with ibrutinib or rituximab in patients with WM.
WM is a rare indolent B-cell lymphoma. Treatment recommendations for WM from current guidelines2 suggest an objective response rate (ORR) of about 80% with contemporary therapies, but they deliver a very low rate of very good partial response or deeper responses (20% or lower), with most patients eventually relapsing or experiencing further disease progression. Furthermore, patients are diagnosed with WM at a median age of 70, when many individuals are intolerant of aggressive therapies because of poor health conditions, hence presenting an urgent clinical need for more effective therapies2.
Preclinical study data of APG-2575 have shown responses generated in WM models resistant or insensitive to ibrutinib, as well as the synergistic effect with ibrutinib in various models of non-Hodgkin’s lymphoma, including follicular lymphoma, diffuse large B-cell lymphoma, and WM.
◆ A Phase Ib study of APG-2575 as a single agent or in combination with chemotherapy for the treatment of patients with r/r AML in China
This multicenter, open-label Phase Ib dose-escalation and dose-expansion study is designed to evaluate the safety and PK of APG-2575 as a single agent or in combination with chemotherapy for the treatment of AML.
AML is the most common form of leukemia in China, with an incidence rate of about 1.62 to 2.32 cases/100,000. AML mainly affects the elderly population, and the median age at diagnosis is 67. The incidence rate of AML increases with age, reaching a peak of 22.5 cases/100,000 in the population aged 80 or older.
Despite recent therapeutic advances in AML, the standard treatment strategy for this condition has remained unchanged, with intense chemotherapy and allogeneic hematopoietic stem cell transplantation (allo-HSCT) as the primary treatment modalities. Because of rapid progression, a high incidence rate, and the complex genetic mutations associated with AML, the rate of long-term survival in patients with AML is still very low, with more than 40% of newly diagnosed patients becoming relapsed or refractory after failing to achieve complete response (CR) during remission induction therapies, or relapse within 6 months after achieving CR. Moreover, an aging population also contributes to the low remission rate, because many elderly patients are intolerant of intensive chemotherapy3.
Preclinical studies of APG-2575 have shown promising anticancer activity and strong synergistic effects with a range of chemotherapies. In addition, Bcl-2 inhibitors in combination with chemotherapies have demonstrated favorable safety and tolerability profiles, suggesting their potential clinical benefits for elderly patients with AML and those who are intolerant of chemotherapies.
“APG-2575 is a key drug candidate in our development pipeline targeting apoptosis, and it is the first China-made Bcl-2 selective inhibitor to enter clinical studies. As a testament to the encouraging preclinical and early-stage clinical data, and Ascentage Pharma’s commitment to its global clinical development strategy, APG-2575 has recently been granted clearances for several Phase Ib/II clinical studies, by the China CDE and U.S. FDA,” said Dr. Yifan Zhai, Chief Medical Officer of Ascentage Pharma. “Combination therapy is playing an increasingly important role in cancer treatment. We hope that these studies will show clinical benefits for patients with relapsed or refractory hematologic malignancies.”
About Ascentage Pharma
Ascentage Pharma (6855.HK) is a globally-focused, clinical-stage biotechnology company engaged in developing novel therapies for cancers, chronic hepatitis B (CHB), and age-related diseases. The company focuses on developing therapeutics that inhibit protein-protein interactions to restore apoptosis, or programmed cell death. On October 28, 2019, Ascentage Pharma was listed on the Main Board of the Stock Exchange of Hong Kong Limited.
Ascentage Pharma has built a pipeline of eight clinical drug candidates, including a novel, highly potent Bcl-2/Bcl-xL inhibitor, as well as candidates aimed at IAP and MDM2-p53 pathways, and next-generation tyrosine kinase inhibitors. The company is conducting 28 Phase I/II clinical trials to evaluate the eight drug candidates in the United States, Australia, and China.
References
1. Slager SL, Benavente Y, Blair A, et al. Medical history, lifestyle, family history, and occupational risk factors for chronic lymphocytic leukemia/small lymphocytic lymphoma: the InterLymph Non-Hodgkin Lymphoma Subtypes Project. J Natl Cancer Inst Monogr 2014;48:41-51.
2. National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN guidelines®). Waldenström macroglobulinemia/ lymphoplasmacytic lymphoma. Version 1.2020 December 6, 2019. Available from: https://www.nccn.org/professionals/physician_gls/pdf/waldenstroms.pdf. Last accessed March 8, 2019.
3. Thol F, Schlenk RF, Heuser M, Ganser A. How I treat refractory and early relapsed acute myeloid leukemia. Blood 2015;126:319-27.
Forward-looking Statement
The forward-looking statements made in this article relate only to the events or information as of the date on which the statements are made in this article. Except as required by law, we undertake no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise, after the date on which the statements are made or to reflect the occurrence of unanticipated events. You should read this article completely and with the understanding that our actual future results or performance may be materially different from what we expect. In this article, statements of, or references to, our intentions or those of any of our Directors or our Company are made as of the date of this article. Any of these intentions may alter in light of future development.
